Abstract
Therapy-related myeloid neoplasm (t-MN) is considered to be a direct stochastic complication of chemotherapy and/or radiotherapy for primary cancer or autoimmune diseases. However, genetic predisposition is reported in 8-12% of sporadic adult cancer patients [Lu et al Nature Communication 2015 and Huang et al Cell 2018]. Similarly, genetic predispositions to t-MN have also been reported in limited single institute studies of small numbers of patients [Churpek et al Cancer 2016].
In this study, we performed comprehensive germline and somatic mutation profiling in t-MN using next generation sequencing. Matched germline material was available for 62/194 (32%) patients. Mutation profiling was correlated with clinical features including family history in 194 patients enrolled in the South Australian MDS (SA-MDS) registry and Cleveland Clinic (CC). An in-house well established filtering pipeline was used for identification of somatic mutations. Only variants with Genome Aggregation Database (gnomAD) minor allele frequency (MAF) of ≤0.01% and variant allele frequency (VAF) of ≥35% were selected for further analysis of germline variants. Variants reported in in the Catalogue of Somatic Mutations in Cancer database and MDS/AML were excluded from further analysis. Variants reported pathogenic in Breast Cancer Information Core (BIC) database and Leiden Open Variation Database (LOVD) were retained. Other variants were included if truncating (nonsense, indels, splice alterations), CADD>20, or predicted deleterious by >4/6 scoring algorithms (GERP>4, PhyloP>2, SIFT, PolyPhen2, MutationTaster and FATHMM).
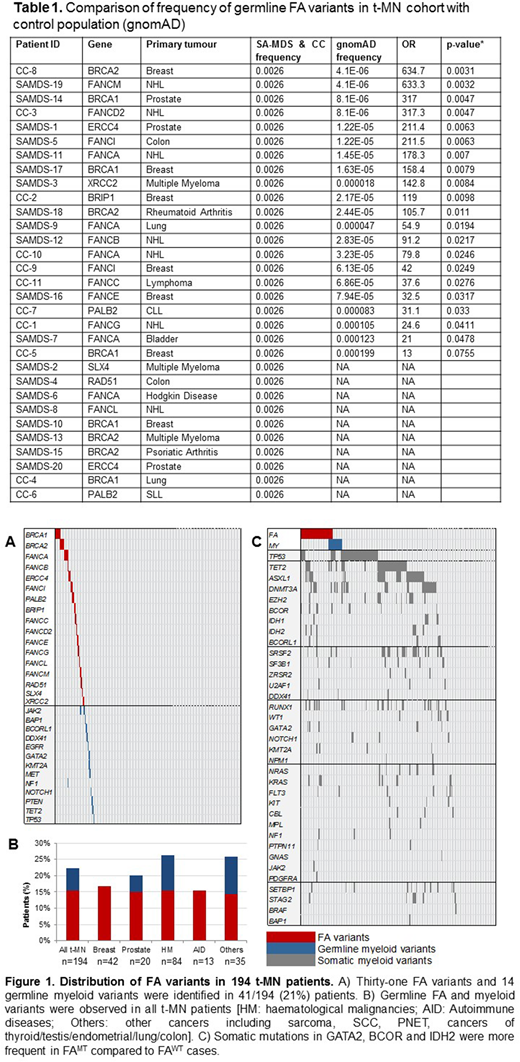
Forty-one (21%) t-MN patients harbored 45 rare (MAF<0.001) and deleterious germline mutations in the Fanconi anaemia (FA) pathway and driver myeloid genes including frameshift indels and splice site alterations in BRCA1, BRCA2, FANCA, PALB2, RAD51, DDX41 and TP53 (Figure 1A-B). The highest number of FA germline variants were seen in BRCA1 and FANCA (n=5 each) followed by BRCA2 (n=4), ERCC4, PALB2 and FANCC (n=2 each). We also identified 14 rare, deleterious myeloid germline variants in 13/194 (6.7%) of t-MN patients. These germline myeloid variants were identified in TP53, DDX41, GATA2 and MET; genes with well-known drivers of myeloid malignancies. Of the five acute lymphoblastic leukaemia patients with t-MN, 2/5 (40%) had rare myeloid germline variants in TP53, GATA2 and KMT2A.
The frequency of these germline mutations in our t-MN cohort is higher than in the general population (gnomAD; Table 1) and in patients with primary malignancies such as breast cancer and lymphoma [Lu et al Nature Communication 2015 and Churpek et al Cancer 2016]. Intriguingly, the frequency of germline FA gene mutations (FAMT) in our therapy-related myelodysplastic syndrome (t-MDS) patients is also higher than those reported in primary MDS patients (18% vs 9%, p=0.02) [Przychodzen et al 2018].
Additionally, of those with available family history, 62% of t-MN patients have first and/or second degree relatives with non-skin cancers. Significantly more patients with FA mutation (FAMT) had first and second degree relatives with cancers compared to patients without FA (FAWT) mutations (82% vs 58%; p=0.03). Additionally, chromosomes 3 and 7 abnormalities, as well as monosomal karyotype, were more frequent in FAMT cases compared to FAWT. Similarly, somatic mutations in GATA2 (10% vs 2%; p=0.02), BCOR (13% vs 4%; p=0.03) and IDH2 (10% vs 2%; p=0.02) were more frequent in FAMT compared to FAWT cases (Figure 1C).
In summary, we show that at least one in five t-MN patients harbor deleterious germline mutations, and 82% of FAMT patients have a first or second degree relative with cancers. These findings have implication in management of not only t-MN patients but genetic testing for their family members.
Branford:Qiagen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cepheid: Honoraria. Maciejewski:Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharmaceuticals, Inc: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharmaceuticals, Inc: Consultancy; Apellis Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy. Hiwase:Celgene: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal